March 1, 2023 report
This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Previously unknown mechanism in precision RNA cleaving by Dicer enzyme revealed
Researchers at the Center for RNA Research, Institute for Basic Science (IBS), in Seoul, have published a study with critical new insights into the structure and function of the Dicer enzyme. Dicer is an enzyme required for the biogenesis of miRNAs and small interfering RNAs (siRNAs), which in turn are drivers of RNA silencing and post-transcriptional regulating of gene expression, one of the bodies' many checks on protein production.
RNA silencing requires efficient processing of double-stranded RNA into miRNAs and siRNAs by Dicer. As the name implies, Dicer takes the larger structure, double-stranded RNA, and cuts it into smaller functioning pieces. The specificity of Dicer's processing has previously only been partially understood, and some of the dicing activity, while functional, needed to be explained.
The study, "Sequence determinant of small RNA production by DICER," has been published in the journal Nature.
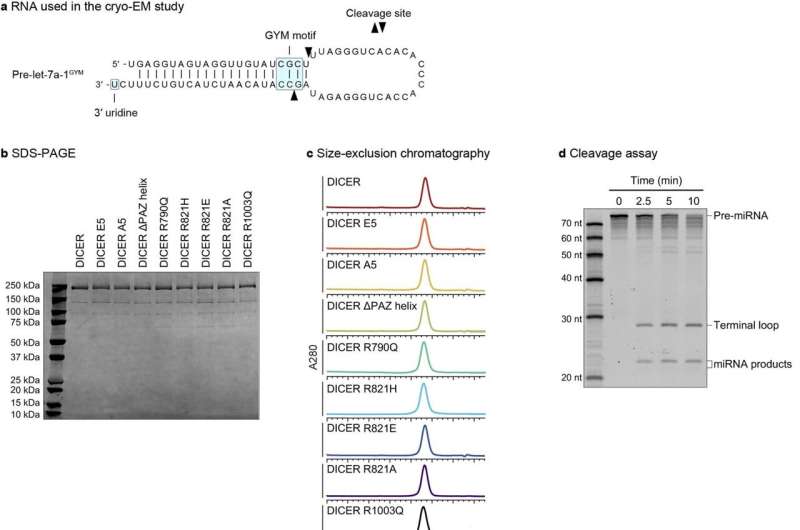
The current research revealed a deeply conserved cis-acting element, termed the "GYM motif," near the cleavage site. This means that when Dicer cleaves precursor RNA molecules to produce miRNA, it can use landmarks (cis-regulatory elements) within the RNA to know precisely where to cut. This mechanism allows Dicer to override the previously identified mechanism of "ruler"-like counting from the 5′ and 3′ ends of pre-miRNA and solves the mystery around how some of the precision dicings take place.
The study employed massively parallel assays with pre-miRNA variants and human Dicer enzyme (DICER1). The researchers selectively altered the GYM motif, assessed the modified Dicer enzyme's ability to process double-stranded RNA, and found it to be "a strong determinant of DICER-mediated processing." The intact GYM motif also improved RNA interference.
The analysis also found that a cancer-associated substitution in Dicer disrupts the recognition of the GYM motif. This discovery could be critically important for future investigations as certain cancers are correlated with increased or decreased Dicer levels, and the association is not currently understood.
Together, the researchers state that their findings "...reveal an integral and conserved mechanism of substrate recognition by DICER and provide a framework to understand how DICER produces small RNAs for biological and therapeutic regulation."
More information: Young-Yoon Lee et al, Structure of the human DICER–pre-miRNA complex in a dicing state, Nature (2023). DOI: 10.1038/s41586-023-05723-3
Journal information: Nature
© 2023 Science X Network